OUR RESEARCH
Brain cells are hardwired together to establish functional circuits between regions. The connected infrastructure enables softwiring by neuromodulators, which fine-tune circuits to help endow the brain with its vast computational power and plasticity. Neuromodulatory signal decoding through G protein-coupled receptors converges downstream to second messengers such as cAMP. Although elegant, this design strategy risks the introduction of errors due to complications in temporal or spatial communication within the neuronal network, which unfortunately can lead to dysfunction and neuronal pathology. Therefore we aim to better understand the molecular logic that underpins neuronal cAMP signal transduction. Our multidisciplinary approach enables a progressive understanding from molecules to cells to circuits.
Molecules
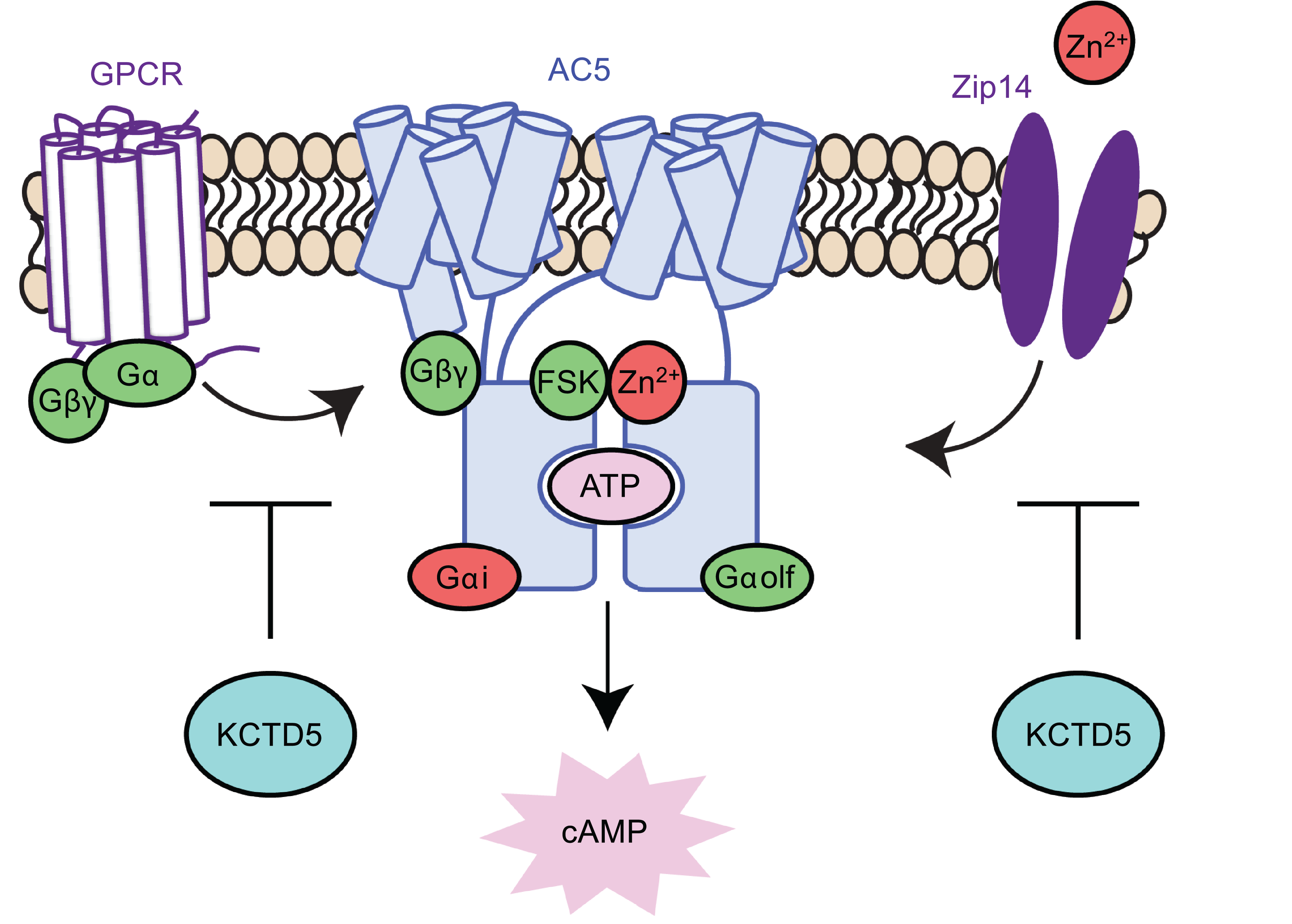
KCTD5 (Potassium Channel Tetramerization Domain 5)
G protein-coupled receptors (GPCRs) initiate an array of intracellular signaling programs by activating heterotrimeric G proteins (Gα and Gβγ subunits). One prominent cascade is G protein regulation of Adenylyl Cyclase Type 5 (AC5)-mediated production of the second messenger cAMP. Therefore, G protein modifiers are well positioned to shape GPCR pharmacology. A current focus is on KCTD5, which exerts its effects as a substrate adapter for Cul3-mediated ubiquitination. Two intriguing targets of KCTD5 include the Gβ subunit and Zip14 (zinc transporter 14). Ongoing projects in the lab are aimed at understanding the details of cAMP modulation by KCTD5.
Cells
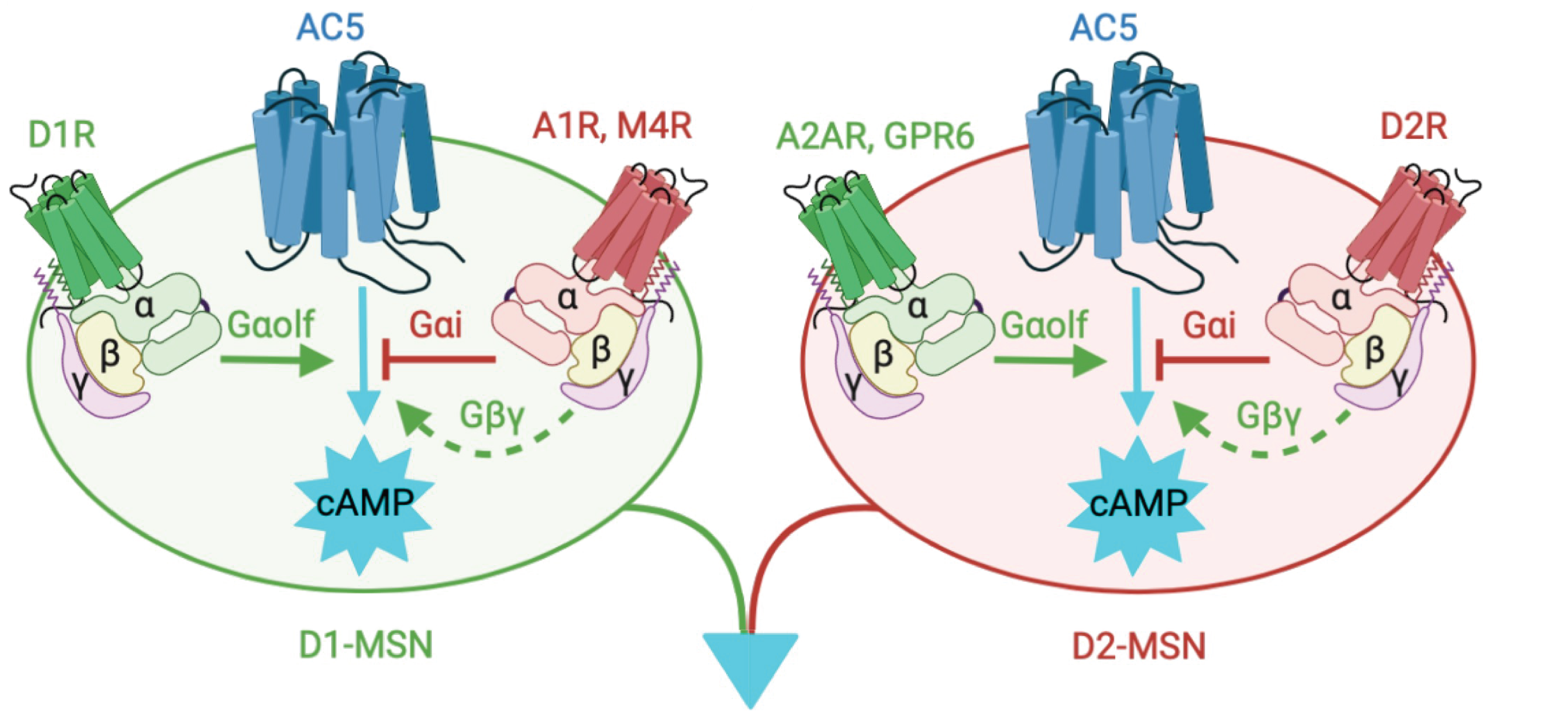
Logic of striatal cAMP signaling
Alterations in striatal cAMP signal transduction contribute to the progression of movement disorders and represent a significant public health concern. Therapeutic options for movement disorders are limited in part by a deficient understanding of the molecular control of cAMP signaling in striatal neurons. Although tremendous progress has been made in unraveling the cAMP pathway, complexity of cAMP regulation in the striatum coupled with the large number of players with pivotal roles not yet fully elucidated has slowed progress towards more effective care strategies. To overcome this barrier, our long-term goal is to better understand the mechanistic nuts and bolts that control striatal cAMP signaling.
Circuits
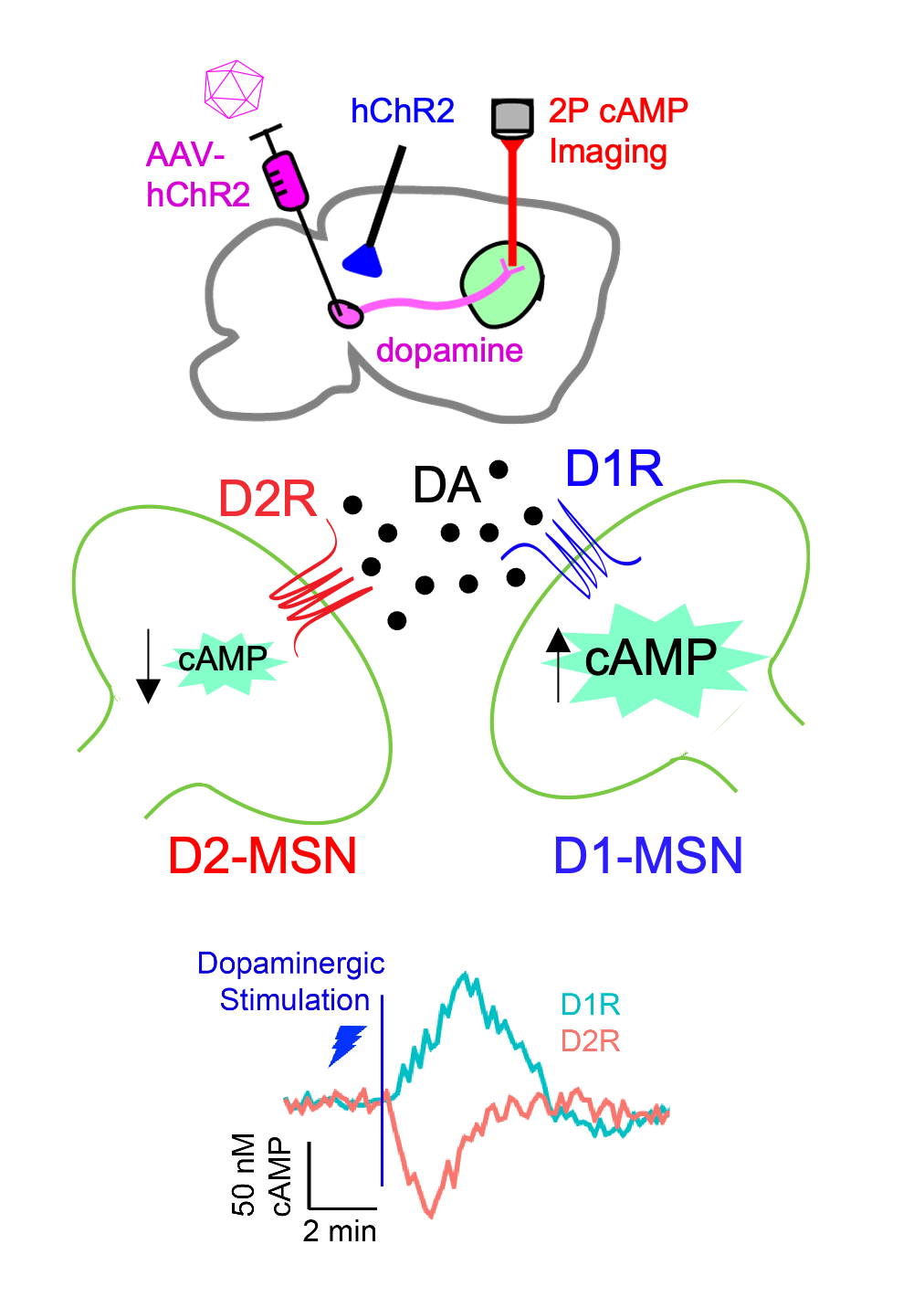
Neuromodulatory signal decoding in striatal circuits
Two distinct striatal circuits are composed of medium spiny neurons (MSNs) hallmarked by differences in Dopamine Receptor subtype expression. Half of the MSNs are enriched with the stimulatory D1 Receptor (D1R; D1-MSN) while the other half express the inhibitory D2 Receptor (D2R; D2-MSN). As a result dopamine increases cAMP in D1-MSNs while decreasing cAMP in D2-MSNs. Although dopamine plays an essential role in motor function, we are still learning about its precise contributions toward cAMP integration in MSN subtypes. To further this understanding, we study the principles of how dopamine neuromodulation adjusts cAMP signal kinetics and sensitivity across intact striatal circuits.

